compressed air testing parameters|validation of compressed air : purchaser The Compressed Air & Gas Institute (CAGI) cites 10 contaminants that typically need to be removed or reduced from low-pressure compressed air used for manufacturing (not breathing air). These contaminants fall into four general categories: 1. Particles (from pipe scale, wear particles and atmospheric dirt) 2. Water . See more Resultado da 19 de jul. de 2023 · 0:00 / 2:59. JOTAPÊ X BRENNUZ O ROUND QUE VIROU MÚSICA 🎵 😱🔥 #batalhasderimas #batalhademcs #batalhasderap. .
{plog:ftitle_list}
web13/11/2023 20h05 Atualizado há 3 meses. Thiago Lopes e Andressa Urach foram casados por dois anos — Foto: Reprodução/Instagram. Andressa Urach, de 36 anos de idade, .
The Compressed Air & Gas Institute (CAGI) cites 10 contaminants that typically need to be removed or reduced from low-pressure compressed air used for manufacturing (not breathing air). These contaminants fall into four general categories: 1. Particles (from pipe scale, wear particles and atmospheric dirt) 2. Water . See moreSelecting ISO 8573-1 as the basis for compressed air quality monitoring and testing is the obvious choice, since it provides a common language that all involved parties can use. ISO 8573 consists of nine parts or sections that address compressed . See moreISO 8573-1 does not include purity classes for gases. ISO 8573 provides testing methods in Part 6 and specifically refers to carbon dioxide, carbon monoxide, hydrocarbons with 5 or less carbons in the chain (C1 to 5), sulfur dioxide, nitric oxide, and nitrogen dioxide. . See more
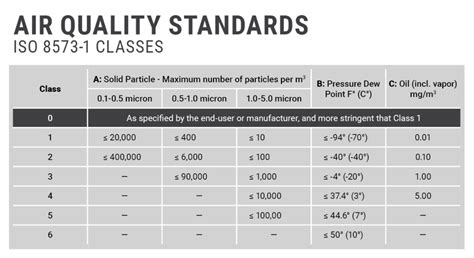
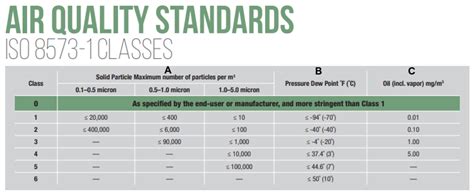
The designation of ISO 8573.1 Purity Classes for compressed air includes the specification name and edition date, followed by the purity . See more
Clean, dry air is typically needed by food, beverage, pharmaceutical, and other industries manufacturing a consumable product. There are no mandated air quality specifications, only general statements, such as: 1. FDA Code of Regulations Title 21, Part 110.40, . See more ISO 8573 consists of nine parts or sections that address compressed air. ISO 8573-1 is the primary section that provides contaminants and purity classes. The other eight sections address sampling techniques and . ISO 8573-7:2003 is the leading standard for testing compressed air. ISO 8573-7 requires before and after blinds, one sterility blank and absolutely no contamination on them. ISO 8573-7 requires a validated impact air .
Auriga Laboratory offers compressed air testing services in PAN India locations for various Industries such as pharmaceutical, food industry etc. Compressed Air Testing. Search for: . We collect the sample through a sampler at an outlet .Start supply of compressed air to the test assembly. 7. If required adjust the flow rate by setting knob of flow meter. 8. Record the time. 9. On completion of 20 minutes stop supply of compressed air to test assembly. 10. Record the reading for oil content shown on the scale by pale blue color. 11. Detach Gastec tube from the test assembly.The air compressor itself can also add contamination, from wear particles to coolants and lubricants. Compressed air storage devices and distribution systems . PARTS 2 – 9 SPECIFY THE METHODS OF TESTING FOR A RANGE OF CONTAMINANTS. ISO 8573.1 : 2001 is the primary document used from the ISO 8573 series as it is this document which specifies .Compressed air isn’t inherently clean; like the ambient environment it’s drawn from, the air in your compressor system is filled with a variety of particles, aerosols, and vapors that can contaminate end processes and products, as well as harm machinery and other equipment.
ISO 8573-4, Compressed air — Part 4: Test methods for solid particle content 3 Terms and definitions For the purposes of this document, the terms and definitions given in ISO 3857-1, ISO 5598, ISO 8573-1 and . The value of air sample parameters (pressure, temperature, air velocity, etc.) shall be within the ranges specified by the test .
validation of compressed air

Regular testing of compressed air systems and other process gases that come into contact with pharmaceutical products is critical to ensuring the quality and integrity of the product. At Trace Analytics, our team of experts is dedicated to providing safe, clean air at the highest quality standards while simultaneously offering guidance for .TRI Air provides equipment to test compressed air samples as part of air quality compliance, safety, SQF. Medical. Solutions for medical gas testing standards including NFPA 99. Fire Service. Compressed breathing air quality for firefighters and SCBA air, NFPA 1989, CGA Grade D, .
5 ton universal materials tensile testing machine
Parameters and limits have to be defined by pharmaceutical users themselves. There are always mistakes, because requirements are phrased inaccurately. . For the test conditions for the water content there are also particular characterstics (maximum 67ppm V/V for compressed gas cylinders or max. 870ppm V/V for compressed air generated by the .In the absence of specified standards governing compressed air quality testing in the manufacturing process or production of pharmaceutical, medical device, and food/beverage applications, it is often best to use composite, site-specific testing programs. This may be the most assured way to produce valid, repeatable testing results that will .oil aerosol in compressed air. This simply alerts you if aerosol is present in your compressed air. A kit is available to order which will allow you (the customer) to take a small air sample and send it to a laboratory for air-testing. Note, this test does not include microorganisms testing. The analysis provided would cover all points in the list
The FA 510 measures the Pressure dew point down to -80°Ctd. Here too, continuous Measuring ensures that an Alarm can be triggered immediately if the compressed air dryer fails. The sensor enables permanent monitoring of the Compressed air dryer. ISO 8573-4 deals with test methods for the solid particle content. Measuring the Free Air Delivery (FAD) of an air compressor can be challenging. With a proper flow meter and some mathematics this task is manageable. This article sheds some light on how to select the flow meter and summarizes parameters to be considered in the FAD measurement task. The ultimate job of an air compressor is to produce compressed air by .Compressed Air Quality Testing Compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air in one form or another. To be a safe, reliable and cost effective utility, compressed air must be treated. Many facilities use international standards to specify the purity (quality) of
usp compressed air requirements
4.3 Testing Parameters: 4.3.1 Analyze compressed air samples for key parameters such as particle count, microbial content (if applicable), moisture content, and oil content. 4.3.2 Use validated analytical methods and equipment (e.g., particle counter, microbial sampler) to determine compliance with defined specifications.Microbial Testing of Compressed Air Micro Testing of Compressed Air or Bioburden Testing per ISO 8573-7 is generally conducted by the pharmaceutical, medical device and food industries. Microbial contaminants found in the compressor or compressed air lines can be devastating to a final product in these industries. A regular Micro Testing program can provide insight to a .
drier be installed (Use TRI test item A82) in the compressed air system. Other gases such as nitrogen may have different system requirements. Microbial testing of air/gas sources is also required per common food and beverage regulatory agencies. Please contact TRI Air Testing, Inc. for further information regarding what needs to be Specification Requirements and Testing Parameters. . Working with a third-party accredited laboratory that specializes in compressed air and gas testing makes the process straightforward. For more information, please contact Trace Analytics via email:[email protected] or phone: .
Employing a Standard for Compressed Air Testing. ISO 8573 consists of 9 parts in which ISO 8573-1 is most frequently cited. Parts 2 through 9 provide analytical techniques and sampling methods. Many air compressor and filter manufacturers cite ISO 8573-1:2010 purity classes to describe the quality of air that can be produced with their products.
The most critical parameters measured under Compressed Air Testing include Particulate Matter, Dew Point, Moisture Content, Oil Content, and Viable Count. At CEGTH, we offer effective testing solutions for your compressed air systems as . Compressed air, also referred to as process gas, is used in many capacities in the pharmaceutical industry. Regular quality control testing plays an important role in the safety of your products. The ISPE Good Practice Guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants and routinely .
Compressed Air Test Parameters: Particulate Testing. Particulate testing measures the concentration of solid particles within the air stream. Common sources of particulates include dust, dirt, and rust from pipes and compressors. These particles can cause blockages, wear, and contamination of end products. Testing methods include particle .flow in the compressed air system. For sampling methods, see ISO 8573-2. Compressed air samples may be routed back into main pipe or vent to the atmosphere after measurement. The value of air sample parameters (pressure, temperature, air velocity, etc.) shall be within the ranges specified by the test equipment manufacturer. 7 Measurement .
Compressed air leak testing helps coffee roaster easily identify energy savings of 10%. Project engineers at a coffee roasting company participated in a pilot study using industrial acoustic imaging technology in a facility where conditions tend to generate many leaks. The test identified the company could save more than 10% per year in energy .
iso guideline for compressed air
%PDF-1.6 %âãÏÓ 17454 0 obj >stream hÞì›[¯$DZ ÿÊ~´ ¤‰[Þ€ :òƒ Ë„(? ‚0–F Š$x Ì ïìÚ½¾&gÆÃáåXÆQ¾ÄÄTDfu¯ŠªZ±;V-{²§Zþä6ö . Compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water concentration, vapor content, oil aerosols, and other pollutants are within limits and do not cause product contamination. . Performing Compressed Air sample testing for Moisture and Oil; Preparation of analysis report and .
Our testing services are designed to meet all your regulatory and testing requirements. If you would like to use our testing services please feel free to contact us through the contact form or call us now on +91-11-45754575. We will be happy to provide you a proposal for Ambient Air Quakity Testing etc.
plastic cup siling test machine universal tester
mini universal tensile test machine micro tensile
Reclama 10 MILHÃO DE MOEDAS GRÁTIS como BÓNUS DE BOAS VINDAS, e começa a rodar os rolos de todas as máquinas de slots neste jogo de casino grátis. Joga com amigos, compete em torneios gratuitos .
compressed air testing parameters|validation of compressed air